AAV Manufacturing Process from GenScript ProBio
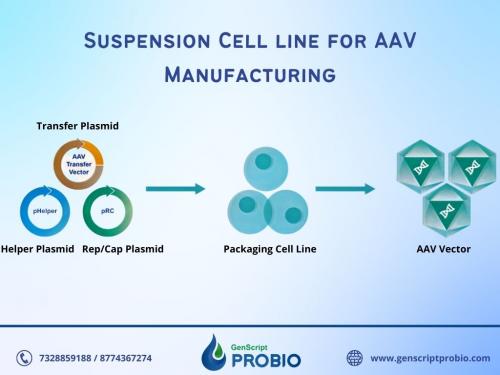 AAV manufacturing is a vital part of gene therapy and gene editing procedures and is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We at GenScript ProBio have grown to be one of the most advanced AAV manufacturers in the world and we are also dedicated to being completely transparent with the process and fully support all of our clients. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs.
AAV manufacturing is a vital part of gene therapy and gene editing procedures and is appropriate for use in non-clinical phases. GenScript ProBio provides AAV manufacturing services suitable for use in non-clinical phases, such as animal studies on primates, toxicology studies, etc. We use a triple transient transfection system, with a manufacturing scale from 5L to 50L. We at GenScript ProBio have grown to be one of the most advanced AAV manufacturers in the world and we are also dedicated to being completely transparent with the process and fully support all of our clients. We follow the phase-appropriate compliance to guarantee the products' safety in using non-clinical phases while reducing time and saving costs.
Advertise on APSense
This advertising space is available.
Post Your Ad Here
Post Your Ad Here
Comments