Understanding the Process of Coal-to-Liquid Fuels
by Rudy P. SysAdmin at howtofindthemoneyABSTRACT
A chemical process used for turning coal into liquid fuels that has the potential for producing hundreds of thousands of barrels per day of hydrocarbon liquids and other byproducts—including electricity—is described. The key to converting coal to liquids is the Fischer-Tropsch (FT) process, which was invented in Germany in the 1920s. This process is used today in full-scale production plants in South Africa and it is being planned for use in plants in many other parts of world. A coal-to-liquids (CTL) industry is highly valued because of the security in using domestic sources of supply (coal) to produce hydrocarbons, in an environmentally acceptable process, that can be blended and refined into liquid fuels and transported to the end-user. In particular, FT fuels can play a significant role in providing a fuel currently used in the transportation industry and thus reducing dependence on imported petroleum and other refined transportation fuel products. This is of particular importance to the United States, which has an abundance of coal.
INTRODUCTION
A coal-to-liquids (CTL) plant is a chemical process plant that converts conventional pulverized coal to carbonaceous liquid fuels and byproduct hydrogen-based gases. These fuels are produced through a process that first converts the coal to coal-gas (or synthetic gas [syngas]) via conventional coal gasification and then converts liquids from the gas via the Fischer-Tropsch (FT) process. Depending on the coal quality and the way the plant is configured and operated, the CTL plant using the FT process can produce significant quantifies of light- to mid-grade, high-value hydrocarbons along with other products such as naphtha, waxes, ammonia, hydrogen, and methane. Coal-to-liquids plants are often designed to produce ~ 2/3 liquid fuels and ~ 1/3 chemicals such as naphtha and ammonia. One of the key products from a CTL plant (that includes postprocessing or refining of FT liquid products) is high-quality/low-sulfur diesel fuel.
The critical components of a CTL plant are the coal gasifier, the enrichment of the synthetic gas to increase the hydrogen/carbon monoxide ratio (H2/CO ratio), and the selected FT process reactor. There are many options for the critical components and component configuration of a CTL plant. In particular, there are at least eight industry-proven gasifiers, primarily used for production of only pipeline-quality natural gas, and at least three commercial production FT processes.
PRODUCING LIQUIDS FROM COAL WITH THE FISCHER-TROPSCH PROCESS
The FT process was developed in the 1920s in Germany. Inventors Franz Fischer and Hans Tropsch developed a process to convert carbon monoxide (CO) and hydrogen (H) to liquid hydrocarbons using iron (Fe) and cobalt (Co) catalysts. The temperature, pressure, and catalyst determine whether a light or heavy liquid fuel is produced. During World War II, petroleum-poor but coal-rich Germany used the FT process to supply its war machine with diesel and aviation fuel after allied forces cut off petroleum imports. Germany’s yearly synthetic oil production reached more than 90 million tons in 1944.
The FT process was (and still is) used to produce most of South Africa’s diesel fuel during that country’s isolation under apartheid. The South African company Sasol Ltd. has produced about 1.5 billion barrels of synthetic fuel from about 800 million tons of coal since 1955 and continues to supply about 28% of that nation’s fuel needs from coal.[1]
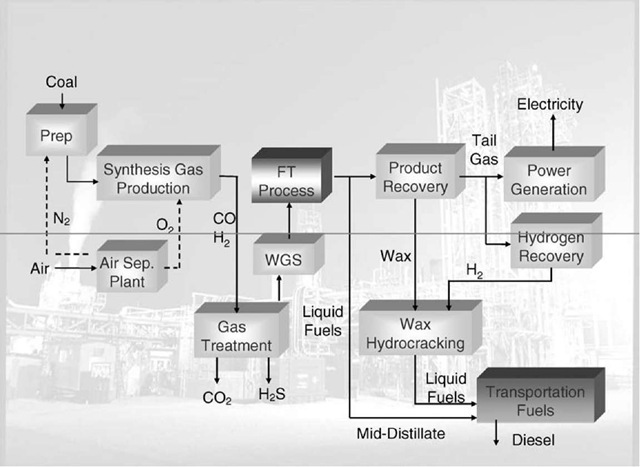
A typical CTL plant configuration using the FT process is shown in Fig. 1. The FT process is comparable with a polymerization process, resulting in a distribution of chain-lengths of the products from the process. In general, the product range includes the light hydrocarbons methane (CH4) and ethane (C2H6), propane (C3H8), butane (C4H10), gasoline (CsH12-Ci2H26), diesel fuel (Ci0H22-Ci5H32), and other long-chained hydrocarbons/waxes (>Ci5). The distribution of the products depends on the FT catalyst used and the process operation conditions (temperature, pressure, and residence time).[2]
The FT process involves the use of slurry-bubble-column (slurry phase) systems using either cobalt-based (Co) or iron-based (Fe) catalysts. With these catalysts, two operating temperatures are available: low and high. For either catalyst, the FT process is exothermic. The FT reaction vessels must be actively cooled to keep them at optimal temperature for the reaction. The heat energy released by the FT process is not at a high enough temperature (200°C-300°C) to drive the production of syngas (the upstream gasification process); but it is adequate for downstream power generation if polygeneration is included in a CTL plant. (Note: polygeneration refers to the production of electricity in combustion turbines from the CTL process waste heat [primarily from the FT process] and tail gas [primarily methane] from the FT process not used to produce desired hydrocarbon products.)
Iron-based catalysts are the preferred catalysts for FT when using low CO/H2 ratio synthesis gases derived from modern coal gasifiers. This is because in addition to reasonable FT activity, FT catalysts also possess high-water gas shift (WGS) activity. In the low temperature range, the iron catalyst can produce 50%-75% wax products. When operating at high temperatures (above 220°C), the gaseous and liquid products are highly olefinic. Another byproduct stream of the FT process is hydrogen gas. Currently, the low-temperature iron FT catalyst produces smaller quantities of products that can be used as chemicals than the high-temperature operation. A drawback with the use of Fe catalysts is their tendency to undergo attrition. This can cause fouling/plugging of downstream filters and equipment, making the separation of catalysts from the oil/wax product very difficult, if not impossible, and resulting in a steady loss of catalysts from the reactor. Iron catalysts have a higher tolerance for sulfur, are less expensive than Co catalysts, and produce more olefin products and alcohols. The lifetime of the Fe catalyst is short and in commercial installations it is generally limited to eight weeks. The Fe catalyst must then be replaced and the spent catalyst disposed of.[3]
A Co-based catalyst gives the benefit of greater management of throughput and output product selection. Co-based catalysts are often utilized because of their high FT activity, C+5 hydrocarbon selectivity, low WGS activity, and relatively low cost compared to Fe catalysts. The Co catalyst produces high-boiling, waxy products when operated in a low-temperature range, but attempts to operate in a high-temperature range (above about 220°C) result in the production of too much methane to be a viable option for producing liquid fuels.
Fig. 1 Process flow diagram of a typical coal-to-liquids (CTL) plant using the Fischer-Tropsch (FT) process.
Cobalt catalysts have the advantage of a higher conversion rate and a longer life (over five years); however, Co catalysts are less tolerant to sulfur and thus the upstream cleaning processes after gasification must remove most of the sulfur from the syngas. In general, the Co catalysts are more reactive for hydrogenation and therefore produce less unsaturated hydrocarbons and alcohols compared to iron catalysts. Processes have been developed to efficiently and cost effectively reactivate/regenerate and reuse a Co catalyst.
CRITICAL DRIVERS FOR A CTL INDUSTRY
There is considerable data—much of it from the oil industry—that indicates the proven reserves of crude oil and natural gas will sustain the world demand for ~ 100 years at current and future predicted consumption rates. There are, however, sufficient coal reserves to sustain world demand for liquid fuels for nearly 200 years. Coal is predicted to last twice as long as the combined proven crude petroleum and natural gas reserves at current usage rates. This is under a projected annual worldwide consumption of coal of 2.2 billion tons/year, or a ~ 1.5%/year increase.[4]
The current and future price of coal is relatively stable compared to other fossil fuels because of coal’s abundance combined with the regional distribution of very large coal reserves. Currently there are about 273 billion short tons (bst) of coal reserves in North America, 173 bst in Russia and the former Soviet Union, 126 bst in China, 93 bst in India, and 90 bst in Australia.[4]
The resurgence in the interest in coal as a viable fossil fuel for direct combustion to produce electricity as well as for the production of liquid fuels is a result of the following energy dynamics:
- Steep increases and unpredictable spikes in crude oil and natural gas prices.
- A decline in domestic oil and natural gas production in those economies with high and increasing energy demands (particularly the United States, China, and India).
- Limitation in current domestic petroleum refining capacity and ability to site and build new refineries.
- The unstable political situation in the oil- and gas-rich Middle East.
- New technology developments in clean coal technologies, including coal combustion and CTL.
In addition, the production of liquid fuels from alternative and indigenous sources is addressed in the Energy Policy Act of 2005 Pub. L. 109-58, and is the linchpin of President Bush’s “Advanced Energy Initiative” goal of new fuel-production technologies to replace more than 75% of our oil imports from the Middle East by 2025.
CTL PRODUCTS: DIESEL FUEL
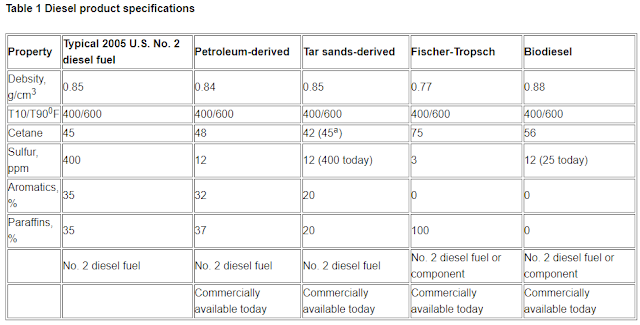
The primary product from a CTL plant is high-quality diesel fuel. The diesel fuel from the FT process will require post-processing to make it compatible and comparable with diesel fuel derived from conventional oil refining. Typical diesel fuel characteristics from several sources are shown in Table 1.
With respect to the production of diesel fuel from a CTL plant, process conditions can be selected to produce maximum amounts of products in the diesel range. However, an even higher yield of diesel fuel can be achieved when the FT synthesis is optimized toward production of wax. Subsequently, the wax can be selectively post-processed (hydrocracking similar to processes in a refinery) to yield predominantly diesel fuel.
The resulting FT fuels from either a Fe or Co catalyst-based process are cleaner-burning than similar fuels from crude oil refining because many of the impurities are removed during the synthesis. The resulting fuels are colorless, odorless, and low in toxicity. Fischer-Tropsch fuels can be used in conventional diesel engines and have improved combustion, which reduces emissions. Fischer-Tropsch fuels have a higher cetane index and less sulfur (<5 ppm), nitrogen oxide, carbon monoxide and particulate matter emissions than petroleum fuels. In addition, the entire coal-to-liquid process is designed to remove other containments typical in fossil fuel combustion, including sulfur and carbon dioxide.
Diesel fuels from a CTL plant are being used as both a neat (a neat CTL diesel fuel is one that is introduced into the distribution system and used directly in a combustion engine without further processing or blending) fuel and as a blending fuel with conventional diesel fuel produced from petroleum processing in a refinery. The blended fuel can help refiners meet current and future sulfur standards as well as stretch the diesel fuel manufactured from conventional petroleum sources. (Note: from 2004 to 2006, the governments in some parts of Japan, Australia, and the European Union [EU] have limited sulfur in highway diesel to no more than 50 ppm. In 2006, the maximum falls to 15 ppm in some parts of the United States. Japan and the EU are expected to further restrict sulfur content to as low as 10 ppm. The cost for refiners to meet these sulfur limitations can range from $1.00 to as much as $3.00 per barrel, thus making the low sulfur diesel from a CTL process a valuable product for blending.)
The market for such fuel would include the domestic transportation industry, the department of defense (DOD), and potentially the agriculture industry. Additional post-FT processing and blending would be required and could produce other fuels, such as JP-4 and JP-8, which could be marketed to the domestic airline industry and DOD. The DOD alone requires over 3,00,000 barrels per day (bpd) of domestic diesel fuel for its operations and desires to supply this requirement with a domestic and secure resource. The Rocky Mountain (Western) states’ usage will require nearly 2,00,000 bpd by 2010 and thus this is another large market. Still another large market for high-quality (low sulfur California air resources board [CARB]) diesel fuel will be California, where predictions show the state consuming over 3 billion gallons/year of diesel by 2010. (Note: current specifications for CARB diesel for use in California will be more stringent than the 2007
U.S. Environmental Protection Agency [EPA] diesel. Fischer-Tropsch diesel will be able to meet these specifications.)
Other products from a CTL plant that can be used in a combustion turbine to produce electricity and steam include elemental sulfur, naphtha, waxes, and tail gas (primarily C4-C6).
FT PROCESSES PROVIDERS
The Sasol Ltd. South Africa FT technology is the most mature full-scale technology in operation today, having been used in South Africa since 1955 and proposed to be used in several international CTL projects. The Sasol II and III CTL plants currently have the capacity to produce 1,50,000 bpd crude oil equivalent liquids.
There are currently three U.S.-based companies with FT technology that can be used in a CTL plant. Each company uses a proprietary technology. However, because there are no full-scale CTL plants in operation in the United States today, none of these three FT technologies have been deployed in a production-scale plant.
Syntroleum develops, owns, and licenses a proprietary process for converting natural gas to synthetic liquid hydrocarbons, known as gas-to-liquids (GTL) technology, as well as CTL technologies. For Syntroleum’s GTL projects, they have executed an agreement with Exxon-Mobile to use ExxonMobile GTL patents to produce and sell fuels from natural gas, coal, or other carbonaceous substances.
Syntroleum is currently developing CTL projects with a recently announced project in Queensland, Australia. The CTL project will include removal and sequestration of CO2 and the production of FT diesel.
Rentech began developing FT-fuel technology in 1981. It has designed FT plants in the range of 2000-40,000 bpd for potential projects in Bolivia, Indonesia, and the United States.
In June 2004, Rentech announced that it had entered into a contract with the Wyoming Business Council to perform engineering design and economic study using Rentech’s patented and proprietary FT technology. The analysis evaluates the economic viability of constructing a mine-mouth plant capable of producing 10,000-12,000 bpd of ultra-low sulfur FT diesel for distribution in Wyoming, California, and other Western states. It is estimated that the facility will require about 3 million tons per year of Wyoming Powder River Basin coal for every 10,000 bpd of fuels production. The study also considered various levels of cogeneration of electric power for sale to the local transmission grid.[5]
Fischer-Tropsch projects based on coal are currently under development in Illinois, Kentucky, and Mississippi. The CTL plant in Kentucky is targeted at 57,000 bpd. In Illinois, Rentech has completed a study to convert an existing natural-gas-based ammonia plant into an integrated plant producing ammonia (900-950 tons/day), 1800-2000 bpd FT fuels, and ~ 10 MW exported electric power using high-sulfur Illinois coal as its feedstock. In Mississippi, Rentech has entered into an agreement with the Adams County Board of Supervisors to negotiate a contract under which Rentech would lease a site for a 10,000-bpd CTL plant producing primarily diesel fuel by 2010.
Headwaters technology innovation group is a wholly-owned subsidiary of Headwaters Incorporated (www. headwaters.com) that promotes and licenses technology for CTL projects using FT technology. Headwaters technology innovation group has developed an iron-based catalyst that is ideally suited for processing coal-derived syngas (synthetic gas) into ultra-clean liquid fuels. Headwaters technology innovation group has patents covering catalyst manufacturing, slurry phase reactor design and operation, production of FT liquids for fuel and chemical feedstocks, and the co-production of ammonia and FT liquids.
CURRENT AND PLANNED CTL INDUSTRY
To date, there is limited experience in full-scale (tens of thousands of bpd) production of liquid fuels from coal. Most experience in full-scale CTL plant operation is with the Sasol Ltd. operation.
A number of pilot-scale (up to hundreds of bpd) plants have been constructed and operated in the United States. These include a 35-bpd plant operating from 1975 to 1985 in Pennsylvania, a 35-bpd pilot plant operated by Air Products and Chemicals in 1984, a 230-bpd plant operated in Colorado by Rentech, a 70-bpd plant in Washington operated in 1998 by Syntroleum/ARCO, and a 2-bpd pilot plant in Oklahoma operated by Syntroleum from 1989 to 1990.
The technology challenge in a CTL plant focuses on the FT process and particularly the FT process fed with gasified U.S. coals. Experience in South Africa is with
South African coal with unique coal qualities and with natural gas as a feedstock GTL. There is a dearth of experience in full-scale operation of a CTL plant in the United States with any FT process, and with the pilot/ demonstration CTL plants, there has been limited testing of the qualities and performance of the fuels.
In addition to the projects noted above, there are a number of planned CTL projects under development around the world, as discussed below.
North America
A number of states are currently either considering or actively developing CTL plants using the FT process, including Kentucky, North Dakota, Mississippi, Missouri, Montana, Ohio, Pennsylvania, West Virginia, and Wyoming. Most of these projects are being developed as consortiums or partnerships of coal companies, gasification/FT suppliers, architect/engineering firms, universities, energy technology centers, and state governments.
Australia
The Australian Power and Energy Limited/Victorian Power and Liquids Project (APEL/VPLP) CTL project was planned in Australia as a joint venture between Australian Power and Energy Limited and Syntroleum. This project includes coproduction of power and hydrocarbon liquids from brown coal in the Labtrobe Valley in the state of Victoria. The initial phase of development envisions a 52,000- bpd plant with CO2 capture and sequestration via subsurface injection.
Asia
Sasol is under discussions with China to build several CTL plants and could also take equity stakes of up to 50% in two proposed Chinese CTL projects. The Chinese proposal is part of a joint venture between Foster-Wheeler/Sasol and Huanqui for two facilities producing 80,000 bbl of liquids per day per site at the Ningxia autonomous region and the Shaanxi province, both in the coal-rich western part of China. An additional CTL/FT demonstration plant of unknown size is being planned by HTIG in Mongolia, China. There are also plans for an 80,000-bpd plant in Indonesia in partnership with Sasol.
India and Pakistan
There is considerable interest from coal companies in India and Pakistan in the Sasol CTL technology. Significant coal reserves in India and Pakistan could help to reduce dependence on imported crude oil.
SITING AND OPERATING CTL PLANTS
The development pathway for CTL plants is uncertain, given the myriad of choices of proven full-scale gasification processes that must be integrated with unproven (on a full-scale) FT processes other than the Sasol Ltd. Fischer-Tropsch process—which has only been used in a fullscale plant with South African coal. Although a CTL plant has the potential for producing tens of thousands of barrels of clean fuel (and other byproducts) in an environmentally-friendly process, there are a number of engineering, infrastructure, and institutional issues that need to be resolved before a full-scale plant is viable in the United States. These include but are not limited to improved materials/catalyst performance and reaction mechanisms for the FT process; permitting, siting, and regulating a first-of-a-kind plant that is neither a conventional coal-fired power plant nor a conventional chemical plant nor a conventional oil refinery; water use and water treatment requirements; securing a coal supply contract under high-demand conditions for coal for power plants; securing an FT-based fuel outtake contract with both a floor and ceiling price; cost-effective carbon capture and other emissions treatment strategies; and optimizing the plant output products that include liquid fuels, naphtha (feedstock for chemical production), ammonia (for fertilizer production), and electricity.
COAL FUEL SUPPLY
In the United States, there are vast deposits of coal— deposits more extensive than those of natural gas and petroleum, the other major fossil fuels. Identified resources include the demonstrated reserve base (DRB), which is comprised of coal resources that have been mapped within specified levels of reliability and accuracy and that occur in coal beds meeting minimum criteria for thickness and depth from the surface that may support economic mining under current technologies.
A typical CTL plant with the capacity of 10,000 bpd would require 10,000-15,000 tons of coal/day. Such a plant operating at 90 + % capacity would consume 3.5-5 million tons of coal/year and produce over 3 million barrels of diesel fuels plus other marketable byproducts. It is anticipated that multiple plants could increase production capacity to 1,00,000 bpd by 2012, 1 million bpd by 2025, and 2-3 million bpd by 2035, and that the coal mining and coal transportation industry would be able to accommodate such production levels.
There are three major coal-producing regions in the United States: Appalachian (primarily Ohio, West Virginia, Kentucky, Tennessee, and Pennsylvania), Interior (which includes the Gulf Region and the Illinois Basin), and Western (which includes the Powder River Basin/ Colorado Plateau and Northern Great Plains).[6]
U.S. coal production in 2004 totaled 1112.1 million short tons and was divided among the regions as follows:
- Appalachian: 389.9 short tons
- Western: 575.2 short tons
- Interior: 146.0 short tons
Of the total coal produced in 2004, 1.016 short tons (91%) were used to generate electricity.[4]
The actual proportion of coal resources that can be mined and recovered economically from undisturbed deposits varies from less than 40% in some underground mines to more than 90% at some surface mines. In some underground mines, much of the coal is left untouched as pillars, required to prevent surface collapse. Adverse geologic features such as folding, faulting, and inter-layered rock strata limit the amount of coal that can be recovered at some underground and surface mines.
COAL MINING AND TRANSPORT
There will be a significant increase in coal mining once the CTL industry matures and production reaches estimated full-scale operation by 2035. An estimated additional 2.54 million tons of coal per day will be required to supply CTL plants operational by 2035. A mature CTL industry would require an additional 2%-3% production increase over 2004 levels and this can be adequately handled by the coal industry. For example, current production in the Western Region’s Powder River Basin is over 350 million tons/year, thus this increased demand would add 8%-12% additional demand if all the additional demand for coal were supplied by this region.[4] This additional demand can readily be provided by the existing mines, thus no new mines would need to be permitted.
There is, however, a current limitation to the amount of coal that can be transported over existing rail lines. Transportation of coal from the mine to the consumer continues to be an issue for the industry. The majority of coal in the United States is moved by railroads exclusively or in tandem with another method of transportation.
A nearby high-speed rail line (and connecting rail spur to the plant) would be required for coal transport capable of transporting ~ 100 coal cars/day for a 10,000-15,000-bpd plant. Petroleum pipelines are the preferred mode of moving FT diesel fuel; however, depending on the location of the CTL plant, rail transportation may also be the best alternative for transporting the diesel and possibly other plant byproducts to a refinery or end-user. A train with 4045 tank cars/day would be required to transport the diesel fuel from a 10,000-bpd plant. Additional transportation modes (tanker truck, tanker car, or pipeline) would be required to haul away other products such as sulfur (truck transport), carbon for sequestration (pipeline transport), ammonia (road or rail transport), and possibly naphtha as a blending stock for gasoline refining (road or pipeline transport). Environmental issues related to increased coal transport (noise, dust) would need to be addressed by each state and community through which the trains would pass.
AIR QUALITY
A CTL plant emits far fewer criteria pollutants into the atmosphere than even the best-controlled and most efficient coal combustion power plant. Sulfur (as H2S) and mercury (as elemental mercury and captured in impregnated activated carbon absorbent) are removed during the gasification. More than 99% of the sulfur and mercury are removed prior to producing liquids in the FT process.
A significant amount of carbon can also be captured as CO2, with the percentage of carbon captured depending on how the plant is operated as well as the economics. Carbon can be further processed for sequestration and the sulfur is converted to elemental sulfur for dry disposal or sale. Carbon dioxide can also be used for enhanced oil recovery or for coal-bed methane extraction.
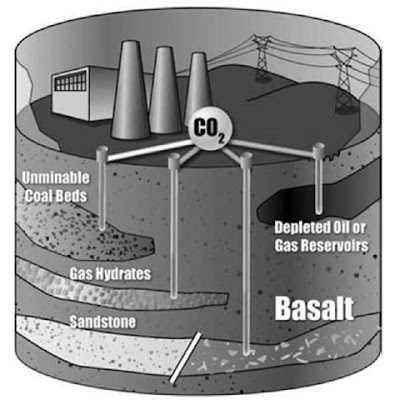
One of the key features of a CTL plant is the potential for substantial carbon capture and sequestration. This can make CTL plants environmentally preferable to combustion plants. Typical strategies for sequestering CO2 include physical trapping, hydrodynamic trapping, solubility trapping, and mineral trapping. These sequestering and use of CO2 strategies are illustrated in Fig. 2.
There are additional emissions of oxides of nitrogen from the combustion process used to generate electricity, chlorine, and particulates from combustion and coal transport, handling, and processing (pulverization). All of these emissions can be treated using the best available control technology (BACT). The goal of control technology for a CTL plant is to reduce the emissions to at least the level of those emitted by a conventional coal-fired power plant using the BACT to meet current air quality standards. Overall, a CTL plant allows for easier and more effective control of criteria pollutants—and additionally CO2—compared to today’s most efficient and controlled coal-fired power plants.
Fig. 2 Illustration of methods of sequestering and using CO2 captured from a coal-to-liquids (CTL) plant.
The only significant air quality issue in the siting and operation of a CTL plant is the potential impact the plant may have on Class I air-sheds such as those in national parks and other designated Class I areas. This would be addressed during the permitting process via air quality dispersion modeling.
WATER QUALITY
A CTL plant using high moisture content coal such as lignite (30%-50% water content) or sub-bituminous (10%-30% moisture content) will likely be a net water producer, depending on whether or not a dry or slurry feed is used for the gasifier (upstream of the FT process) and whether or not a significant amount of (excess) power is produced requiring a cooling tower. Under the plant design scenario using 30% moisture content coal, a net production of 100-200 gal/min would be discharged for a 10,000-bpd liquids plant. This water would require treatment and disposal in surface or underground wells.
The 10 largest coal producers and exporters in Indonesia:
Sponsor Ads
Created on Jul 26th 2019 22:22. Viewed 766 times.
Comments
No comment, be the first to comment.
Sponsored
Tags Links
More Articles
- Replacing Gasoline With Coal-to-Liquid Fuels - Coal is the Future!
- Benefits of Coal Ash
- Technology of Coal Direct Liquefaction for Production of Synthetic Hydrocarbon Mixture
- The Competitive Race to Produce Synfuels Using the Coal-To-Liquid (CTL) Process
- Turning Low Calorie Coal (Brown Coal) into Liquid or Gaseous Fuels
Similar Articles
- The Process of Producing Liquid Fuels From Coal
- Turning Low Calorie Coal (Brown Coal) into Liquid or Gaseous Fuels
- Replacing Gasoline With Coal-to-Liquid Fuels - Coal is the Future!
- Coal Is Liquid Fuels
- West Virginia's Coal-to-Liquid Fuels Project Will be Completed by Early 2023