Is Ethanol (C2H5OH) Polar or Nonpolar?
The widespread use of ethanol has led many alcohol consumers to wonder why ethanol 96 is such a good disinfectant and disinfectant. So, is ethanol 96 alcohol polar?
Is Ethanol (C2H5OH) Polar or Nonpolar?
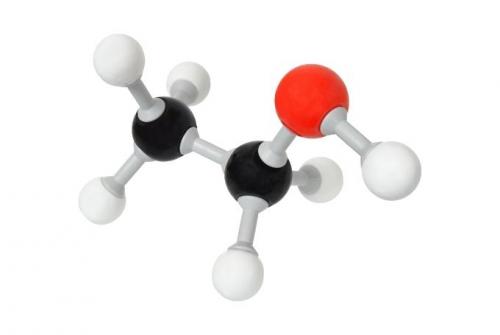
Ethanol is a non-polar chemical. Although there is a small region of it that remains polar, most of the solvent ethanol 96 is nonpolar making it oil soluble.
Molecular morphology of ethanol 96
Leaving aside some puzzling laws of physics, don't worry. Quang Trung Chemicals won't ask you to go back and re-read your high school textbooks.
Instead, I'll show why ethanol's polarity is important and how it allows the chemical to do its job for everything from cleaning up messes to killing bacteria.
Polarity of 96 alcohol products and why it matters
Most people have a bottle of alcohol at their office or garage. Given the few other cleaning chemicals, alcohol 96 products are extremely cheap and effective.
It is also a very versatile solvent that is useful for many different things, including:
surface disinfection.
Alcohol kills mildew.
Use alcohol to clean the glass
grease cleaning.
Remove old car wax and prepare paint.
…and some more!
While rubbing alcohol is undeniably effective and versatile, almost no one really understands the principle or why rubbing alcohol can do it. And this is why Quang Trung Chemical wants to send you this article. All are related to molecular polarization.
What is a polarizer?
The most basic way to imagine the polar part of a molecule is to think of a traditional magnet. In a magnet, one side has a positive charge and the other side holds a negative charge.
This is why magnets attract each other and why metals stick to magnets. In a similar way, the atomic shape of a molecule determines whether it is polar or not:
In some polar molecules, one or more atoms "gather" the majority of electrons, resulting in one side of the molecule being positively charged, while the other side is negatively charged.
Water molecules are made up of (+H) and (-OH) which is a good example of a polar chemical
For a less polar substance, a few atoms that are in equilibrium with some electrons should carry a negative charge.
In other words, in some polar molecules, most of the positively charged electrons jump onto another element, leaving some of the other elements electron deficient (or negatively charged).
In contrast, in most nonpolar solvents, most electrons are able to swap places and move from one element to another, but they will never cause one side of the compound to lose electrons.
how to distinguish polar and non-polar chemicals?
In terms of geometry: the majority of polar molecules, polar chemicals have asymmetrical shape (displaced to one side) while most nonpolar molecules have a symmetric molecular shape.
Another way is to determine by electronegativity difference. In other words, a moment when the electronegativity difference of the molecule > 0.4 will be a polar bond and < or = 0.4 a nonpolar bond.
polar substances dissolve some polar substances
This term describes the ability of two liquid chemicals to mix together and form a homogeneous chemical.
Take for example oil, water and alcohol. Oil is nonpolar, water is polar and alcohol is mostly nonpolar. If you want to mix oil and water together, you know what will happen; The oil floats into strange little bubbles on the surface of the water. In other words, polar and non-polar chemicals cannot mix.
Now, try dissolving the alcohol with the oil; you will find that the thickness of the oil quickly breaks down and is likely to be easily wiped off. This means that oil and alcohol are miscible.
If you remember your high school chemistry class, you will remember that: "polar chemicals dissolve most polar substances, non-polar substances dissolve most non-polar chemicals".
This means that to dissolve a non-polar chemical like oil, you will need another non-polar solvent such as alcohol. This is why you can't clean the oil residue with plain water (it will only make it messier).
Are all alcohols non-polar?
I came across this question while browsing Reddit the other day. The answer is, YES. All alcohols have the same basic chemical morphology and molecular properties.
Alcohol is a polar solvent that plays a necessary role in most industries
The main difference is the method used to make the alcohol. But in general, ethanol or even denatured alcohol, industrial alcohol is also effective in dissolving some other undigested substances.
Reading this far, you may wonder: "Alcohol is a non-polar substance, so why is alcohol soluble in water?".
Why is alcohol soluble in water?
The reason is that alcohol belongs to the category of being nonpolar (which allows it to dissolve and dissolve another non-polar chemical that is oil-based). So alcohol SHOULD not be miscible with water, right?
Yes, ethanol alcohols are mostly nonpolar. However, that “most” is what makes the difference. Unlike a COMPLETELY non-polar solvent, most alcohol molecules have a polar fraction.
This is what allows them to be mixed with water.
In other words, alcohol is defined as a less polar chemical or a "dipolar chemical". Both polar and non-polar.
In fact, if the electronegativity shows a strong polarity or a low polarity of a chemical, it is natural that there will be some solvents that belong to the group of substances between the polar and nonpolar boundaries.
One caveat: The higher the percentage of alcohol, the more non-polar the solvent and the better it can remove oil.
How does ethanol alcohol kill germs and bacteria?
The combination of some of the alcohol's non-polar and polar molecules is what makes it so easy to kill bacteria and viruses.
Unlike many other germicidal solvents on the market, ethanol is non-toxic and can quickly eliminate deadly bacteria in seconds. This is because bacterial structures are both polar and non-polar molecules.
When alcohol comes into contact with bacteria, most nonpolar and polar molecules react with each other. It breaks the shape of the bacteria and effectively "kills" the bacteria.
There are countless ways to kill bacteria before they've actually started entering your body. And using alcohol is just one of a few simple, safest ways for people's health.
Advertise on APSense
This advertising space is available.
Post Your Ad Here
Post Your Ad Here
Comments