IVF Laboratory Equipment by Shivani Scientific Industries
 Pre-implantation genetic screening-PGS (also known as aneuploidy screening) involves checking the chromosomes of embryos conceived by in vitro fertilisation (IVF) or intra-cytoplasmic sperm injection (ICSI) for common abnormalities.This avoids having abnormal embryos transferred to the womb during IVF or ICSI.PGS is accomplished as part of the in vitro fertilization (IVF) process,commonly used to treat infertile couples.But,in this case,the embryos are tested for the presence or absence of specific chromosomal abnormalities prior to transfer into the uterus (IVF-PGS).
Pre-implantation genetic screening-PGS (also known as aneuploidy screening) involves checking the chromosomes of embryos conceived by in vitro fertilisation (IVF) or intra-cytoplasmic sperm injection (ICSI) for common abnormalities.This avoids having abnormal embryos transferred to the womb during IVF or ICSI.PGS is accomplished as part of the in vitro fertilization (IVF) process,commonly used to treat infertile couples.But,in this case,the embryos are tested for the presence or absence of specific chromosomal abnormalities prior to transfer into the uterus (IVF-PGS). The Embryology IVF laboratory has to be designed in such a way that it provides an environment that is as close to optimum as possible for the growth of human embryos and to provide the best resulting pregnancy rates for patients undergoing IVF treatment or cycles.For the success of any infertility or fertility clinic the inbuilt ivf laboratory plays an important role. IVF laboratory is the key to success in In Vitro Fertilisation(IVF) clinic.Maintaining air quality control is the biggest challenge for all IVF laboratories and IVF clinics.Embryos and specimens may be subjected to unforeseen and unexpected harsh environmental contamination such as mix of dust,pesticides,fire smoke,volcanic ash,mold,and bacteria.Apart from maintaining high level of air quality,experience of Fertility specialists,Fertility embryologists and scientists are the key factors to be kept in mind for the success of any IVF lab.
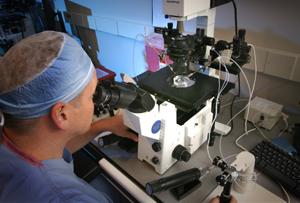
The IVF Lab Facility is the most critical work area in the entire ART Centre.In this area sperm,embryos and Oocytes are manipulated openly after following disinfection and sterilization steps.Pre implantation genetic screening is also done at this step only in ivf lab.Once the embryos are created in ivf lab,after 2-3 days until the cells have divided and the embryo consists of about eight cells,they are tested for any chromosomal abnormalities.A trained embryologist removes one or two of the cells (blastomeres) from the embryo.The chromosomes are examined to see how many there are and whether they are normal.One,two or three of the embryos without abnormal numbers of chromosomes are transferred to the womb so that they can develop.Any remaining unaffected embryos can be frozen for later use.
Hence the ambience required for embryos to grow must be of high and standard quality.Air quality,particle count and sterility are extremely important in an IVF laboratory to ensure best clinical outcomes.Human embryos are extremely sensitive to the presence of any smells,Volatile Organic Compounds (VOC),Chemically Active Compounds (CAC). The minimal requirement for any laboratory offering ART is to implement a quality control system for all the embryologists.It has been proved that air quality is crucial for the success of IVF because of the presence of volatile organic compounds (VOCs),microbes,and perfumes,all of which can be harmful to embryo development in vitro.Therefore IVF laboratories are equipped with high efficiency particulate air (HEPA),and activated carbon filters plus positive pressure for air particulate control,with or without CODA system.
The requirement of embryos to develop in the IVF laboratory in the initial stages is very crucial and important as it requires maximum care and hygienic environment. The equipment that is used during the IVF process must provide an optimum environment for embryos while they are growing in the lab.While the embryos are within the incubators a proper environment is provided by a controlled temperature and gas mixture.The gas levels and temperature of each incubator are checked daily.The removal of the embryos from the incubator and placement on the microscope for examination is performed only when absolutely necessary.While the embryos are out of the incubators they are placed on surfaces that are heated to body temperature just like the incubators.Their time out of the incubator is for a minimal time and the air quality within the room prevents their exposure to air borne toxins.The importance of the culture system and how it is used to maximize growth cannot be overstated.
The clean room in an IVF Laboratory should be built in such a way that outside contaminates cannot enter the lab.The clean room area is the most important place as the embryos originate here and the success of the ivf cycle and in turns PGS depends on it.Experience of Fertility specialists, Fertility embryologists and the scientists are the key factors to be kept in mind while setting up a state-of-the-art IVF lab.You need IVF lab designer,who are driven by passion of success to ensure continuous development and delivery of State of the ART IVF Projects.
Quality is the Key to Reliable IVF procedures.Shivani Scientific Industries (P) Ltd. is highly focused company on Assisted Reproductive Technologies and Bio-Medical Equipments with proven track record of over 42 years.Shivani has contributed into development of ART field in India significantly.We supply complete range of IVF laboratory consumables and IVf laboratory equipment such as laminar flow work stations,anti vibration tables,temperature controlled equipment and air filtration equipment.We represent 26 International Companies into India.We also have our own Fornax range of innovative IVF products which are exported to more than 30 countries.We specialises in providing Turnkey Project Services for setting up of IVF Labs,OT rooms,ICU,CCU,NICU complying to various regulatory standards.We have recently started a new Division for IVF consumables which brings world class products such as Wallace range of medical devices and Life Global range of Culture Media. Amongst other brands are NUNC,MTG,Fornax,purten & graf,Merck,GMS,Blue star,RI consumables and others.
You are Welcome to the world of Shivani Scientific where end to end solutions are provided for enabling life through technology at http://www.shivaniscientific.com/ or at http://ivfworld.wordpress.com or http://www.shivaniscientific.com/ivfproducts/spermfuge.htm. You can contact us on 91 22 2896 1768 to inquire about any IVF laboratory equipments and consumables.
Advertise on APSense
This advertising space is available.
Post Your Ad Here
Post Your Ad Here
Comments