Approval for Abortion Pill – FDA
FDA / Food and Drug
Administration allowed the approval to make use of RU - 486 Abortion Pill. This
decision by FDA / Food and Drug Administration has brought out the victory in
the ongoing battle since 12 years case for brining the concept and approving it
as early abortion method in the country. On the pill, the proponents stated that there
has been usage of the medicine by millions of women and in around 13 countries.
The update states that the medicine shall be made available even with the
doctors within a month’s time. However
it is approved for the States, it is seen that in France, the case had made a
debut in the year 1988 and there had been certain restrictions on the anti-
abortion organizations have pledged to actually continue the fight on this
case.
Following the case,
there is timely solution ensuring that the pill be consumed as prescribed and
with every essential safety measures. FDA / Food and Drug Administration has
made the mandate section that in case of such medical abortion, women be
provided with certain brochures such as MedGuides in which the medical
procedures for conducting the process becomes easy and the eligibility of usage
is made clear thus providing a complete procedural steps to the users. The
guide to also to allow women what to expect during the procedure of consuming Online Abortion Pills, the side effects
of the pill, what to expect during the procedure of medically aborting the
unwanted pregnancy and the necessary tips one should keep to undergo in case of
any emergencies.
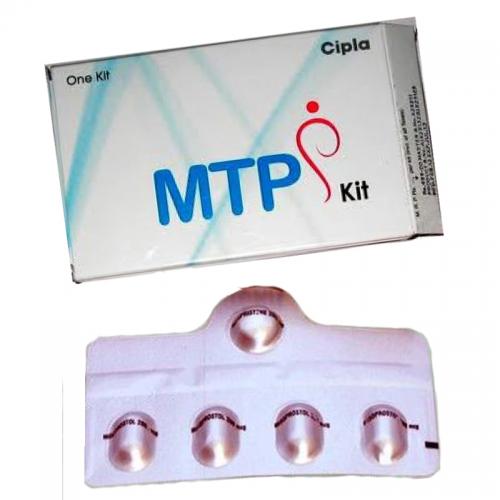
RU-486, is now known as
Mifepristone and this medicine guided to be consumed within a period of 49 days
from the beginning of the last menstrual period. Women are guided to consume 4
Misoprostol pills within a period of 24 to 48 hours from consumption of Mifepristone
which is the primary medicine.
The guidance factor involves
one using the medicine orally while the secondary dose – Misoprostol can be
consumed vaginally also. It is recommended that users consume the medicines
orally for the better results. Women are guided to consider consuming the
medicine at a convenient place and if required with assistance. However, the
guide will be made available to users for better assistance. A follow up on the
case can be conducted with doctors or at a clinic incase of any requirement /
emergency. As per medical survey and analysis, the medicine can successfully
end the unwanted case of pregnancy.
The Food and Drug Administration will now allow “Mifepristone” to be provided
to doctors for distribution and the mandate to involve distribution to only the
trained doctors so as to have accurate diagnosis of the situation and also
understand the duration period of pregnancy, detect if the case is ectopic or
tubal in nature as it is noted and advised that for women with such pregnancy,
the medicine Mifepristone is not recommended. The FDA / Food and Drug
Administration has also made few restriction to use Mifepristone such as in
case of severe bleeding or there is any other case where in the surgical
abortion can be performed to end the complex case, during such cases, the
surgeon can make arrangements and provide care to the person.
As per the analysis, the medicine Mifeprex is safe and effective in ending the
early pregnancy pressures by causing blockage of the progesterone hormone’s
action and equally maintaining safety. The advantage of the medicine is that
there is safety involved completely.
What to expect during the process?
Users can expect cases
such as painful level cramping, bleeding, feeling nauseated, guilt feel, loss f
mood and fatigue during the consumption of Mifepristone. The pill induces a
miscarriage condition that causes the termination to occur. Procedure includes
blocking of the hormone that is essential for pregnancy and the development.
As per the proponents pushed to the FDA / Food and Drug Administration, the pill
should be made as a active and assisting alterative to the surgical methods
thus reducing the worries of every woman who is finding trouble during such
situations. FDA’s decision on it amidst the election campaigning can be a case
of fierce response and controversy. However, the news is that NAF / National Abortion Federation’s members have
made a preparation to offer the medicine Mifeprex and is already into training
the physicians to how to make use of the pill.
Post Your Ad Here
Comments