Coal have different quality characteristics
by Rudy P. SysAdmin at howtofindthemoney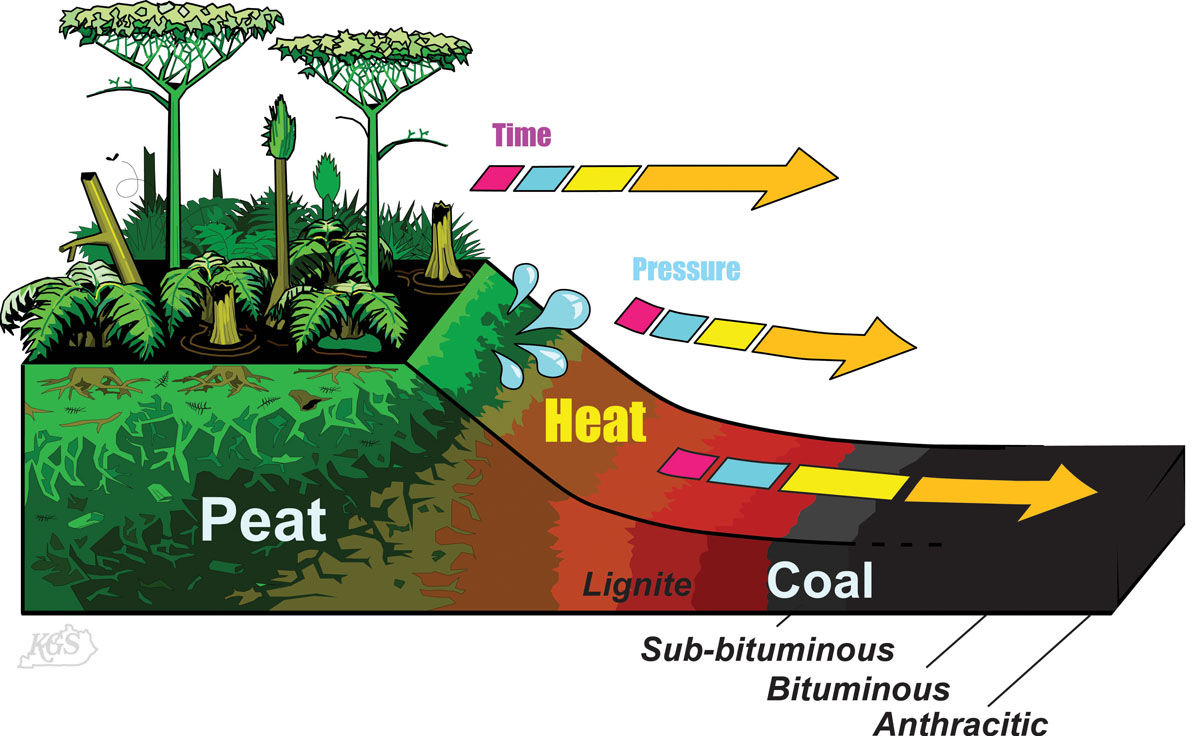
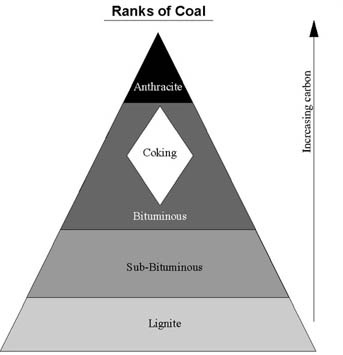
The stages of physical and chemical changes that proceed from peat through coal are called coalification, and are classified and described by rank. In general, rank categories are defined based on recognizable changes in coal parameters. Slightly different categories and parameters are used in different countries, but in general, proceed in increasing rank from lignite, to sub-bituminous, to bituminous, to anthracite coal. Different ranks of coal cannot always be differentiated based only on their physical appearance. Testing of different parameters is often needed to differentiate coal ranks.
Different ranks of coal:
1. Lignite Coal
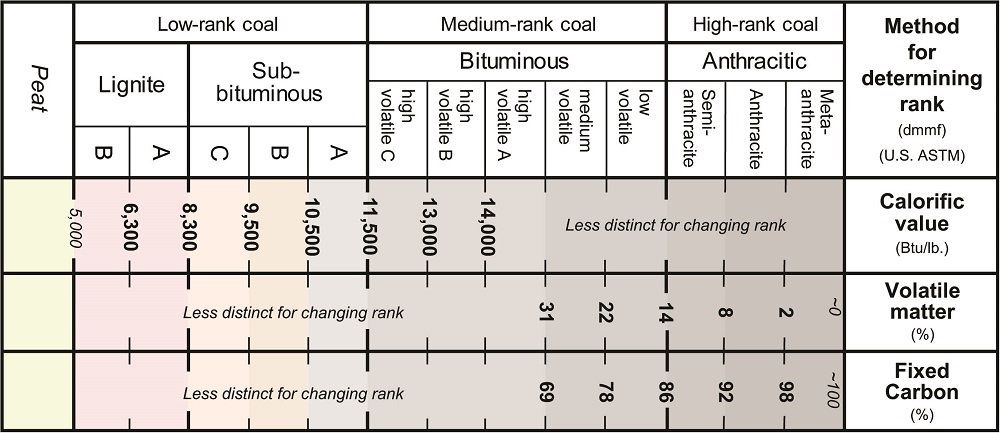
Lignite is the lowest rank of coals. Lignites are brown in color and have an earthy, crumbly texture. They look more like dirt, than what people normally think of when they think of coal. In the U.S. rank classification, lignites are defined based on calorific value. Lignites have calorific (heating) values less than 8,300 Btu/lb (ASTM, 2014). In some other countries, lignites are defined based on their moisture content. In other classifications, vitrinite reflectance (%Ro) may be used to define lignites. Vitrinite reflectance in lignites is generally %Ro less than 0.4 (UNECE, 1998; Alpern and DeSousa, 2002; ISO, 2005).
Physical and Chemical Changes (Lignite Rank)
The dominant processes during the lignite stage of coalification are physical compaction, dewatering (sometimes called dehydration) and biogeochemical gelification. Biochemical gelification is the biological and chemical breakdown of carbon molecules in the lignite (Stach et al., 1992; Teichmüller, 1989). These processes are part of the broader phases of diagenesis, and eodiagenesis in coalification (see figure).
Moisture content decreases dramatically from peat through lignite rank, through expulsion of water and through changing coal chemistry (the loss of certain chemical functional groups) resulting from biochemical gelification (Barnes et al., 1984; Levine, 1993; Mukhopadhyay, and Hatcher, 1993). At the peat to lignite transition, lignite commonly has moisture contents of 60%, but moisture decreases to approximately 20% in subbituminous coals (Teichmüller and Teichmüller, 1982; UNECE, 1998).
Biogenically-sourced methane (CH4) and carbon dioxide (CO2) are also expulsed from lignite during the chemical and physical changes that accompany gelification (Stach et al., 1982; Levine, 1993).
An important physical change in coal during the lignite rank of coalification is the formation of organized sets of fractures or joints, called cleats. Cleats are what give coal its common blocky appearance. They are also pathways for moisture and gas movement and subsequent mineralization (Stach et al., 1992, Laubach et al., 1998).
2. Sub-Bituminous Coal
Sub-bituminous coals are the second division of low-rank coals. They are transitional between lignite and bituminous coal. In the U.S. rank classification, sub-bituminous coals and their subdivisions (A, B, C) are supposed to be based on calorific value. By ASTM standards, sub-bituminous coals have calorific (heating) values of 8,300 to 11,500 Btu/lb (ASTM, 2014). In practice, however, there is considerable overlap between what is called a higher rank sub-bituminous coal (subbituminous A) and a lower rank bituminous (high volatile C) coal. For example, coal beds of the Illinois Basin (and western Kentucky) are considered high volatile C bituminous coals although they have calorific values of less than 11,500 Btu/lb. Vitrinite reflectance (%Ro) in subbituminous coals is generally Ro between 0.4 to 0.5%Ro. As with calorific values, reflectance values for what are considered bituminous C and subbituminous A coals in the United States overlap.
Sub-bituminous C (lower rank sub-bituminous) coals in the U.S. rank system are brown and earthy like lignites, whereas, sub-bituminous A (higher rank sub-bituminous) coals are gray to black and shiny like bituminous coals. Sub-bituminous coals are sometimes referred to as black lignite (Jackson, 1997).
Physical and Chemical Changes (Sub-bituminous Rank)
During coalification of sub-bituminous coals, moisture loss continues, but biochemical gelification is replaced by geochemical gelification, also termed vitrinization. Vitrinization is the process by which low-rank components of coals called huminite macerals are transformed into vitreous (shiny) vitrinite macerals, which are characteristic of higher rank coals. Vitrinization is followed by bituminization, in which biogenic breakdown of the coal matrix is replaced by thermal breakdown (Mukhopadhya and Hatcher, 1993; Levine, 1993; Teichmüller, 1989; Stach et al., 1982). Above the rank of sub-bituminous B coals, most of the changes to coal chemistry and structure are driven by increased thermal (heating) rather than biogenic processess. The phase of increased thermally-driven changes is termed catagenesis in maturation studies (e.g., Tissot and Welte, 1984).
3. Bituminous Coal
Bituminous coals are black, shiny, and generally hard. They are a medium-rank coal. Bituminous coals generally have calorific values above 11,500 Btu/lb and volatile matter below 14% (ASTM, Jackson, 1997). In the Illinois Basin (and western Kentucky), however, the lower rank end of what are termed bituminous coals in the United States have calorific values of 11,100 to 11,300 Btu/lb. The international coal classification system uses vitrinite reflectance (%Ro) to subdivide bituminous coals. In this system, bituminous coals have %Ro between 0.5 and 1.9. As for calorific value, reflectance values for what are considered bituminous C and subbituminous A coals in the United States overlap.
In the U.S. classification system, bituminous coals are divided into five subdivisions. High-volatile bituminous coals (high volatile B and C) are classified using gross calorific (heating) value. High volatile bituminous C coals have calorific values of 11,500 to 13,000 Btu/lb. High volatile bituminous B coals have calorific values of 13,000 to 14,000 Btu/lb. Above the high volatile bituminous rank, however, calorific values are less distinctive for increasing rank. Medium and low volatile bituminous ranks in the U.S. system, are defined based on volatile matter or fixed carbon rather than calorific value. Medium volatile bituminous coals have 22 to 31% volatile matter on a dry mineral-matter free basis. This corresponds to a fixed carbon content of 69 to 78%. Low volatile bituminous coals have 14 to 22% volatile matter on a dry mineral matter free basis. This corresponds to a fixed carbon content of 78 to 86% (ASTM, 2014).
Physical and Chemical Changes (Bituminous Rank)
Bituminous coals begin with abundant volatile matter (high volatile bituminous coals) but with increasing rank, volatile matter is lost forming medium and then low volatile bituminous coals (e.g., Stach et al., 1982). Chemically, the process of bituminization continues through the bituminous rank. The term “bituminous” comes from the increase in bitumen at this rank. Bitumen is petroleum tar or natural asphalt. Heating coal to the rank of high bituminous coal is approximately equivalent to the maturation level in organic-rich source rocks at which oil is generated (Dow, 1977; Teichmüller, 1974; 1989; Tissot and Welte, 1984; Levine, 1993; Mukhopadhyay and Hatcher, 1993). Bitumen is petroleum tar or natural asphalt.
Coalification from the upper high volatile A bituminous through low volatile bituminous ranks is dominated by thermal degradation and “cracking” of the molecular components of the coal and expulsion of thermogenic methane (methane evolved from heating). The process of cracking, also called “repolymerization” essentially breaks down complex carbon compounds into simpler carbon compounds. This process is part of the catagenesis phase of maturation. Thermogenic methane generation begins in the early catagenesis stage, but peak methane expulsion from the coal matrix occurs in mid-catagenesis, in medium and low volatile bituminous ranks (Dow, 1977; Hunt, 1979; Teichmüller, 1974; 1989; Tissot and Welte, 1984). This is why some medium volatile bituminous and higher rank coals are gassy underground. Methane is flammable, so cautions are taken in underground mines to monitor and prevent the buildup of methane leaking from higher rank coal beds to prevent underground mine explosions.
4. Anthracite Coal
Anthracitic coals are high-rank coals. They are shiny (glassy) and break with a conchoidal (glass-like) fracture. Most coals do not reach anthracitic rank, which requires high heat from very deep burial, tectonic metamorphism, or contact metamorphism with igneous intrusions. The anthracitic rank is divided into three parts; semi-anthracite, anthracite, and meta-anthracite. In the U.S. rank classification system, anthracitic ranks are defined based on volatile matter and fixed carbon contents. Anthracitic coals have volatile matter less than 14% and fixed carbon contents greater than 86% on a dry mineral-matter free basis (ASTM).
Anthracite, which is a specific rank of anthracitic coals, has volatile matter from 2 to 8%, and fixed carbon contents of 92 to 98% on a dry mineral-matter free basis (ASTM; Jackson, 1977).
Physical and Chemical Changes (Anthracitic Rank)
Anthracitic rank marks the beginning of distinctive physical and chemical changes to coal termed anthracitization, graphitization, metadiagenesis, and telodiagenesis in different reports (see figure). The most distinctive physical change at anthracite rank, is the development of conchoidal fracture and the loss of cleats in coal. Conchoidal fractures are curved, reflective, glass-like surfaces. Chemically, anthracites show a sharp decrease in hydrogen content and the hydrogen to carbon (H/C) ratio relative to medium- and low-rank coals. At the molecular level, coalification at anthracitic ranks involves condensation of aromatic carbon-ring systems to larger ordered-carbon structures (Stach et al., 1982; Krevelen, 1993; Levine, 1993). This results in a very ordered, carbon-rich (98 to <100% C) coal at the meta-anthracite rank.
Beyond Anthracite?
It was once thought that continued heating and burial of anthracite coal would result in the formation of the mineral graphite (a crystalline form of carbon), and then even diamond; the purest, most-ordered crystalline form of natural carbon. However, recent research suggests that the activation energy for the formation of graphite may be too high to be formed in nature just by normal geologic burial heating processes. Instead, it appears that considerable strain energy (as occurs in mountain-building areas of continental crust) are likely needed to transform anthracites into graphite (Wilkes et al., 1993; Bustin et al., 1995).
The 10 largest coal producers and exporters in Indonesia:
Source:  Kentucky Geological Survey
Kentucky Geological Survey
Sponsor Ads
Created on Jul 10th 2019 01:58. Viewed 491 times.
Comments
No comment, be the first to comment.



