Gasification Introduction | Coal Gasification Encyclopedia
by Rudy P. SysAdmin at howtofindthemoneyGasification is a technological process that can convert any carbonaceous (carbon-based) raw material such as coal into fuel gas, also known as synthesis gas (syngas for short). Gasification occurs in a gasifier, generally a high temperature/pressure vessel where oxygen (or air) and steam are directly contacted with the coal or other feed material causing a series of chemical reactions to occur that convert the feed to syngas and ash/slag (mineral residues). Syngas is so called because of its history as an intermediate in the production of synthetic natural gas. Composed primarily of the colorless, odorless, highly flammable gases carbon monoxide (CO) and hydrogen (H2), syngas has a variety of uses. The syngas can be further converted (or shifted) to nothing but hydrogen and carbon dioxide (CO2) by adding steam and reacting over a catalyst in a water-gas-shift reactor. When hydrogen is burned, it creates nothing but heat and water, resulting in the ability to create electricity with no carbon dioxide in the exhaust gases. Furthermore, hydrogen made from coal or other solid fuels can be used to refine oil, or to make products such as ammonia and fertilizer. More importantly, hydrogen enriched syngas can be used to make gasoline and diesel fuel. Polygeneration plants that produce multiple products are uniquely possible with gasification technologies. Carbon dioxide can be efficiently captured from syngas, preventing its greenhouse gas emission to the atmosphere and enabling its utilization (such as for Enhanced Oil Recovery) or safe storage.
Gasification offers an alternative to more established ways of converting feedstocks like coal, biomass, and some waste streams into electricity and other useful products. The advantages of gasification in specific applications and conditions, particularly in clean generation of electricity from coal, may make it an increasingly important part of the world's energy and industrial markets. The stable price and abundant supply of coal throughout the world makes it the main feedstock option for gasification technologies going forward. The technology's placement markets with respect to many techno-economic and political factors, including costs, reliability, availability and maintainability (RAM), environmental considerations, efficiency, feedstock and product flexibility, national energy security, public and government perception and policy, and infrastructure will determine whether or not gasification realizes its full market potential.
The graphic below is a representation of a gasification process for coal, depicting both the feedstock flexibility inherent in gasification, as well as the wide range of products and usefulness of gasification technology.
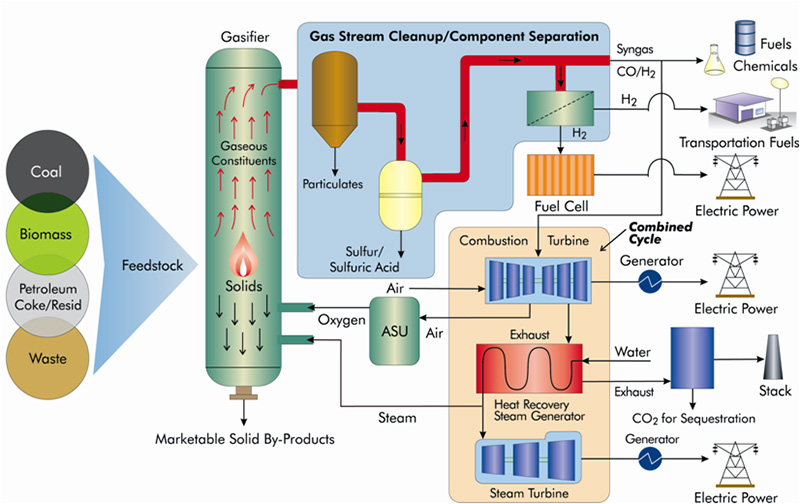
FUNDAMENTALS
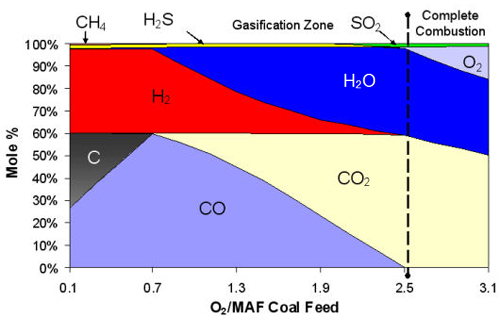
Gasification is a partial oxidation process. The term partial oxidation is a relative term which simply means that less oxygen is used in gasification than would be required for combustion (i.e., burning or complete oxidation) of the same amount of fuel. Gasification typically uses only 25 to 40 percent of the theoretical oxidant (either pure oxygen or air) to generate enough heat to gasify the remaining unoxidized fuel, producing syngas. The major combustible products of gasification are carbon monoxide (CO) and hydrogen (H2), with only a minor amount of the carbon completely oxidized to carbon dioxide (CO2) and water. The heat released by partial oxidation provides most of the energy needed to break up the chemical bonds in the feedstock, to drive the other endothermic gasification reactions, and to increase the temperature of the final gasification products.
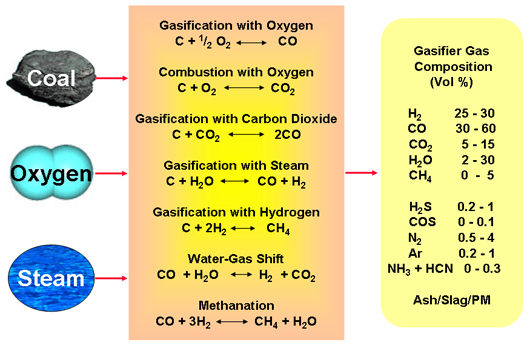
REACTIONS & TRANSFORMATIONS
The chemistry of gasification is quite complex and is accomplished through a series of physical transformations and chemical reactions within the gasifier. Some of the major chemical reactions are shown in the diagram below. In a gasifier, the carbonaceous feedstock undergoes several different processes and/or reactions:
- Dehydration – Any free water content of the feedstock evaporates, leaving dry material and evolving water vapor which may enter into later chemical reactions.
- Pyrolysis – This occurs as the feedstock is exposed to rising temperature in the gasifier. Devolatization and breaking of the weaker chemical bonds occurs, releasing volatile gases such as tar vapors, methane, and hydrogen, along with producing a high molecular weight char which will undergo gasification reactions.
- Combustion – The volatile products and some of the char react with limited oxygen to form carbon dioxide (CO2), carbon monoxide (CO), and in doing so, provide the heat needed for subsequent gasification reactions.
- Gasification – The remaining char reacts with CO2 and steam to produce CO and hydrogen (H2).
- Water-gas-shift and methanation – These are separate reversible gas phase reactions taking place simultaneously based on gasifier conditions. These are minor reactions which play a small role within in the gasifier. Depending on the desired product, the syngas may undergo further water-gas shift and methanation processing downstream from the gasifiers.
DETAILED GASIFICATION CHEMISTRY
The chemical reactions of gasification can progress to different extents depending on the gasification conditions (like temperature and pressure) and the feedstock used. Combustion reactions take place in a gasification process, but, in comparison with conventional combustion which uses a stoichiometric excess of oxidant, gasification typically uses one-fifth to one-third of the theoretical oxidant. This only partially oxidizes the carbon feedstock. As a "partial oxidation" process, the major combustible products of gasification are carbon monoxide (CO) and hydrogen, with only a minor portion of the carbon completely oxidized to carbon dioxide (CO2). The heat produced by the partial oxidation provides most of the energy required to drive the endothermic gasification reactions.
Within a gasification process, the major chemical reactions are those involving carbon, CO, CO2, hydrogen (H2), water (steam) and methane (CH4), as follows:
The combustion reactions:
1. C + ½ O2 → CO (-111 MJ/kmol)
2. CO + ½ O2 → CO2 (-283 MJ/kmol)
3. H2 + ½ O2 → H2O (-242 MJ/kmol)
Other important gasification reactions include:
4. C + H2O ↔ CO + H2 "the Water-Gas Reaction" (+131 MJ/kmol)
5. C + CO2 ↔ 2CO "the Boudouard Reaction" (+172 MJ/kmol)
6. C + 2H2 ↔ CH4 "the Methanation Reaction" (-75 MJ/kmol)
With the above, the combustion reactions are essentially carried out to completion under normal gasification operating conditions. And, under the condition of high carbon conversion, the three heterogeneous reactions (reactions 4 to 6) can be reduced to two homogeneous gas phase reactions of water-gas-shift and steam methane-reforming (reactions 7 and 8 below), which collectively play a key role in determining the final equilibrium synthesis gas (syngas) composition.
7. CO + H2O ↔ CO2 + H2 "Water-Gas-Shift Reaction" (-41 MJ/kmol)
8. CH4 + H2O ↔ CO2 + 3 H2 "Steam-Methane-Reforming Reaction" (+206 MJ/kmol)
In the low-oxygen, reducing environment of the gasifier, most of the feedstock’s sulfur coverts to hydrogen sulfide (H2S), with a small amount forming carbonyl sulfide (COS). Nitrogen chemically bound in the feed generally converts to gaseous nitrogen (N2), with some ammonia (NH3), and a small amount forming hydrogen cyanide (HCN). Chlorine is primary converted to hydrogen chloride (HCl). In general, the quantities of sulfur, nitrogen, and chloride in the fuel are sufficiently small that they have a negligible effect on the main syngas components of H2 and CO. Trace elements associated with both organic and inorganic components in the feed, such as mercury, arsenic and other heavy metals, appear in the various ash and slag fractions, as well as in gaseous emissions, and need to be removed from the syngas prior to further use.
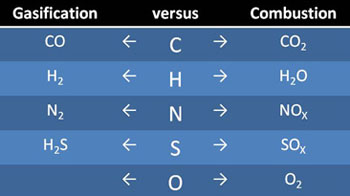
The table summarizes the main transformations of solid fuel constituents to gaseous species in both gasification and combustion. This shows clearly the marked differences between gasification (resulting in syngas) and combustion (resulting in exhaust gas).
THERMODYNAMICS AND KINETICS
Gasification reactions are reversible. The direction of the reaction and its conversion are subjected to the constraints of thermodynamic equilibrium and reaction kinetics. The combustion reactions
C + ½ O2 → CO (-111 MJ/kmol)
CO + ½ O2 → CO2 (-283 MJ/kmol)
H2 + ½ O2 → H2O (-242 MJ/kmol)
essentially go to completion (to the right). The thermodynamic equilibrium of the gasification reactions
CO + H2O ↔ CO2 + H2 "Water-Gas-Shift Reaction" (-41 MJ/kmol)
CH4 + H2O ↔ CO2 + 3 H2 "Steam-Methane-Reforming Reaction" (+206 MJ/kmol)
are relatively well defined and collectively impose a strong influence on the thermal efficiency and the produced syngas composition of a gasification process. Thermodynamic modeling has been a useful tool for estimating key design parameters for a gasification process, for example:
- Calculating of the relative amounts of oxygen and/or steam required per unit of coal feed.
- Estimating the composition of the produced syngas.
- Optimizing the process efficiency at various operating conditions.
Other deductions concerning gasification process design and operations can also be derived from the thermodynamic understanding of its reactions. Examples include:
- To produce a syngas with a low methane content, a high temperature and substantial amount of steam in excess of the stoichiometric requirement are required.
- Gasification at very high temperature, on the other hand, will increase oxygen consumption and decrease the overall process efficiency.
- To produce a syngas with a high methane content (see discussion of synthetic natural gas production), gasification needs to be operated at low temperature (~700°C), but the methanation reaction kinetics will be poor without the presence of a catalyst (see discussion of catalytic gasification).
- There is considerable advantage to carry out gasification under pressure. At a typical entrained flow gasifier operation temperature of ~2,700°F (1,500°C), the syngas composition shows very little change as a function of operating pressure (Higman, 2008), but significant savings in compression energy and cost reduction from using smaller equipment can be realized.
Relative to the thermodynamic understanding of the gasification process, its kinetic behavior is more complex. Very little reliable kinetic information on coal gasification reactions exists, partly because it is highly depended on the process conditions and the nature of the coal feed, which can vary significantly with respect to composition, mineral impurities, and reactivity. Certain impurities, in fact, are known to have catalytic activity on some of the gasification reactions.
SYNGAS COMPOSITION
The figure of gasification reactions and transformations illustrated the concept of coal gasification, and noted resulting composition of syngas. This can vary significantly depending on the feedstock and the gasification process involved; however typically syngas is 30 to 60% carbon monoxide (CO), 25 to 30% hydrogen (H2), 0 to 5% methane (CH4), 5 to 15% carbon dioxide (CO2), plus a lesser or greater amount of water vapor, smaller amounts of the sulfur compounds hydrogen sulfide (H2S), carbonyl sulfide (COS), and finally some ammonia and other trace contaminants.
The Wabash facility, using E-Gas™ gasification technology, operated on a variety of fuels over this period including two different types of coal and petroleum coke. Syngas composition remained relatively constant despite changes in coal composition. Although the gasifier is capable of handling a wide range of feedstocks, variations from the coal used as the design basis for the system can reduce syngas and steam production (differences in production will depend on the coal feed and how it differs from the design feed). Sudden changes in feedstock can also cause disruptions in other plant processes. The syngas H2:CO ratio is relatively high (>0.7), typical of a slurry-feed gasification system. The high levels of COS during the earlier years of operation were a result of problems with the COS-hydrolysis unit (specifically, catalyst poisoning by trace metals and chlorides and surface area degradation by overheating). These problems were remedied over the course of the project1.
Syngas Composition Variability
The linked table summarizes data from several demonstrations, analyses, and reports on syngas composition across a variety of gasifiers, and types of coal feedstocks, showing the wide variations that occur. Caution must be used in examining this table, as many factors may be missing that have significant impact on the syngas produced. For example, to accurately detail effects on syngas composition a detailed account of the coal used (ultimate and proximate analysis) would be required. Gasifier operating mode, operating conditions, etc., would also need to be specified (for example, the GE gasifier can be operated in three different modes: total quenched; radiant & convective cooling; radiant followed by partial quenched—the syngas could be quite different for each). This table, therefore, serves as a general overview and is not detailed enough for design work. See the linked sources for information on the development of this table.
The data shows that a wide range of syngas compositions can be obtained by varying the gasifier type, feedstock, and operation parameters. The typical design process of a gasification facility entails selecting a readily available feedstock for the potential facility site and a suitable gasification technology, and then using variations on downstream processes to optimize the syngas composition for creating the desired end product.
SYNGAS OPTIMIZED FOR INTENDED PRODUCTS
The discussion of syngas composition is of considerable importance considering the varying requirements on composition and impurities demanded according to final uses of the syngas. The following table shows the widely varying characteristics desirable for the principal uses of syngas, including use as fuel gas to fire boilers or turbines in power cycles, use of syngas as feedstock for production of synthetic fuels such as gasoline, use as feedstock for methanol synthesis, and use as feedstock for production of hydrogen.
The 10 largest coal producers and exporters in Indonesia:
Sponsor Ads
Created on Jul 12th 2019 01:55. Viewed 2,585 times.
Comments
No comment, be the first to comment.



