CO2 Capture Using Membranes Holds the Promise of Cost Efficiency
by Rudy P. SysAdmin at howtofindthemoneyClimate breakdown caused by carbon dioxide (CO2) emission into the atmosphere is a major existential problem confronting humanity. The most acceptable solution would be complete termination of the use of fossil fuels, or at least fast reduction of their use by all countries, in line with the Paris agreement. This will ensure the planetary warming is limited by 2 degrees C. Emission reductions are slow, however, and most countries are unlikely to reach the goals of the agreement.
Technological solutions for massive CO2 emissions prevention are therefore critically needed. Some technologies for CO2 capture, for instance, sorption by liquid amine chemicals, are already mature enough to be applied at scale. However, they are costly and come with a burden of toxic chemical disposal once they lose their CO2 binding property. Alternative technologies are therefore of a great importance.
Separation of gasses with the help of membranes is emerging as a key technology for the establishment of a sustainable society. Wide deployment of membranes can help to capture huge amounts carbon dioxide emitted in industrial processes. In contrast to conventional CO2 capture, gas separation with membranes holds the promise of cost efficiency. However, to achieve economical CO2 capture at mass scale, the membranes need several critical features: fast CO2 transport through their structure; high CO2 selectivity (i.e., to be a less permeable barrier for other gasses); mechanical strength and chemical resistance. Additionally, membranes should be composed of materials that are inexpensive at mass production, so organic polymers (conventional plastics and rubbers) are most attractive for industrial applications.
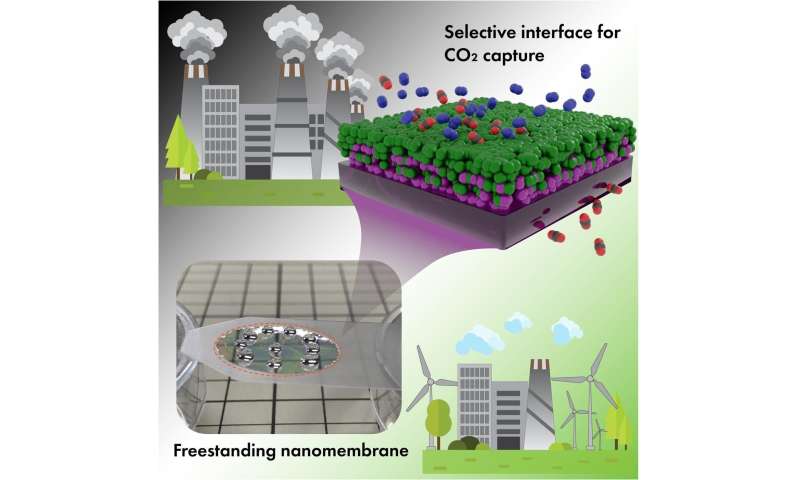
Thin-film composites represent a specific architecture of membranes to provide a robust structure for industrial applications. These membranes, which contain multiple functional layers (made of organic polymers), offer a good solution for large-scale CO2 capture. However, even benchmark organic polymers with the best separation performance (high CO2 permeability and high CO2/N2 selectivity) do not yet exhibit satisfactory separation performance because of their inability to form sufficiently thin, defect-free and mechanically stable membranes.
In a new study, researchers report for the first time how ultimately thin selective layers with a thickness of several nanometers can be used to achieve desired separation properties. They used well-known polymers for the study—polyether block amide (Pebax-1657) as selective layer and polydimethylsiloxane (PDMS) as a gutter layer. They examined what happens with the gas separation property when thickness of the selective layer was pushed to the extreme of several nanometers. They report that when a selective layer of separation membranes becomes very thin, it can form a specific interface with the gutter layer in a composite structure. This nanoscale interface delivered unexpectedly high selectivity towards CO2. Gentle and ultra-short plasma treatment of the hydrophobic PDMS layer that is needed to promote adhesion with the hydrophilic selective layer revealed itself as a tool to control and tune the activity of the molecular interface between two polymers.
They found that this interface made a determining impact on the CO2 selectivity. Together with high permeation rates enabled by low thickness, the membranes fit nicely into the area of the separation properties needed for industrial CO2 capture (e.g., post-combustion capture at fossil fuel power plants). These results open a new and unexplored area of interface-governed gas separation that can be used by engineers to design more efficient membranes for variety of useful applications.
The 10 largest coal producers and exporters in Indonesia:
Source: NETL
Sponsor Ads
Created on Jul 15th 2020 02:21. Viewed 555 times.
Comments
No comment, be the first to comment.



