Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin (a hormone that regulates blood sugar) or when the body cannot effectively use the insulin it produces. There are three major types of diabetes mellitus (DM)—type 1, type 2, and gestational diabetes. Type 1 diabetes mellitus (T1DM), insulin-dependent diabetes mellitus, or juvenile-onset diabetes may account for about 5% of all cases of diabetes. T1DM is characterized by a genetic predisposition manifested in one of several human leukocyte antigens. Type 2 diabetes mellitus (T2DM)
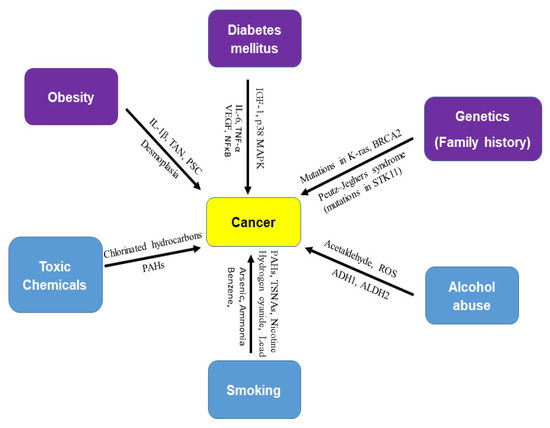
, non-insulin-dependent diabetes mellitus, or adult-onset diabetes, account for about 90% (285 million people), and this number is projected to grow to 438 million by 2030. T2DM is a form of diabetes mainly characterized by high blood glucose, insulin resistance, and relatively a weaker insulin-stimulated response under hyperglycemic conditions. In obese individuals with euglycemia, peripheral insulin resistance is present but compensated by increased insulin secretion [
18,
19,
20]. Insulin resistance progressively worsens in predisposed individuals, along with progressive β-cell dysfunction and the reduction of β-cell mass, eventually leading to T2DM [
18,
19,
20]. DM is not only related to retinopathy, neuropathy, nephropathy, and cardiovascular diseases but is also related to several liver diseases such as nonalcoholic fatty liver disease (NAFLD), steatohepatitis, and liver cirrhosis. Long-standing T2DM, insulin resistance, and obesity have been shown to modestly increase the risk of several types of cancers [
21,
22,
23,
24,
25,
26,
27,
28]. According to the World Health Organization, obesity is defined by the body mass index of more than 30 kg/m
2, and overweight is 25–30 kg/m
2. Obesity causes chronic inflammation of the body and is a risk factor for many cancers. There are a number of conditions associated with diabetes, such as thyroid disease, coeliac disease, polycystic ovary syndrome, diabetes insipidus, necrobiosis lipoidica diabeticorum, mastopathy, muscular conditions, dental health complications, and certain types of cancer. Growing evidence suggests that patients with colorectal, breast, liver, endometrial, and gastric cancers and leukemia [
29,
30] who also have DM are at increased risk of cancer recurrence and mortality [
31].
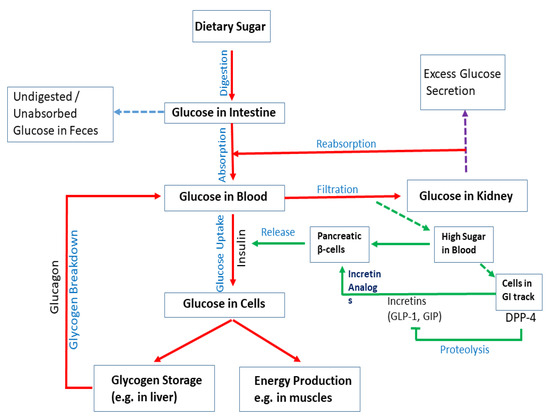
Upon digestion of dietary sugar, glucose is absorbed by the intestine, which results in a rise in the blood glucose level (). In the liver, insulin regulates glucose production/utilization. When glucose levels increase in the blood and insulin is secreted by pancreatic β-cells. Intestinal cells secrete DPP4, which inhibits the production of incretins such as GLP-1 and GIP; they act on pancreatic β-cells to regulate insulin production. In physiological states, the combined action of glucagon and insulin allows the precise regulation of hepatic glucose output. Although glucagon induces hepatic glucose production, insulin acts as a potent inhibitor of glucose production when its concentration in the blood rises. In addition to inducing glycogen synthesis, insulin also inhibits hepatic glucose production. In the case of insulin resistance, physiologic levels of circulating insulin are insufficient to elicit the appropriate insulin response in hepatic cells. In the liver, insulin resistance impairs glycogen synthesis, fails to suppress glucose production, enhances lipogenesis, and increases the synthesis of proteins. Insulin resistance occurs due to a decrease in the metabolic response of insulin-responsive cells to insulin or, at a systemic level, an impaired/lower response to circulating insulin by blood glucose levels. In skeletal muscle, insulin resistance is considered to be an important extra-pancreatic factor in the development of T2DM. Under physiological conditions, insulin stimulates muscle glycogen synthesis by enhancing glucose uptake from plasma.
Figure 2. Mechanisms of diabetes. Upon digestion of dietary sugar, glucose is absorbed by the intestine, which causes an increase in the blood glucose level. Glucose levels increase in the blood and insulin is secreted by pancreatic β-cells. Insulin enhances uptake of glucose into cells. Glucose is stored as glycogen in the liver or utilized for energy production in muscles. If blood sugar levels are low, glucagon breaks down glycogen in the liver to release glucose and increase glucose levels. Intestinal cells secrete DPP4, which inhibits the production of incretins (e.g., GLP-1 and GIP). They act on pancreatic β-cells to regulate insulin production. Although glucagon induces hepatic glucose production, insulin acts as a potent inhibitor of glucose production when its concentration in the blood rises. Undigested/unabsorbed glucose is excreted from the body.